


- Arabic
- Czech
- English
- French
- German
- Hindi
- Hungarian
- Indonesian
- Italian
- Japanese
- Korean
- Persian
- Polish
- Portuguese
- Romanian
- Russian
- Spanish
- Tagalog
- Thai
- Turkish

Acid Conductivity Measurement Tools Precision Testing Solutions
Acid Conductivity Measurement Tools Precision Testing Solutions
Did you know 32% of chemical plants report production delays due to inaccurate acetic acid measurements? Temperature fluctuations alone can skew conductivity readings by 18-22%, costing manufacturers an average of $47/minute in wasted reactants. Your current sensors might be leaking profits through invisible gaps in data accuracy.

(acid electrical conductivity)
Precision That Adapts: Next-Gen Acid Conductivity Sensors
Our AX9 series tackles the twin demons of acid electrical conductivity
monitoring: thermal drift (
| Feature | AX9 Pro | Competitor X |
|---|---|---|
| Temp Compensation | ±0.5% | ±2.1% |
| Response Time | 0.8s | 3.2s |
Battle-Tested in Extreme Conditions
When PharmaCorp needed to monitor acetic acid electrical conductivity at 85°C with ±0.1 mS/cm accuracy, our custom solution delivered 99.4% batch consistency. Their ROI? 14 months payback period through reduced chemical waste.
Your Customized Monitoring Strategy
Whether you're handling:
- ✅ High-purity acetic acid (5-100% concentration)
- ✅ Temperature swings (-20°C to 150°C)
- ✅ Explosive atmospheres (ATEX Zone 1)
Stop Guessing, Start Measuring
Our clients achieve 91% faster troubleshooting and 23% lower operating costs within 6 months. Ready to join them?
Claim your FREE conductivity audit and discover how much you're losing to measurement errors. Limited slots available!

(acid electrical conductivity)
FAQS on acid electrical conductivity
Q: What factors influence the electrical conductivity of acids?
A: The electrical conductivity of acids depends on their ionic dissociation and ion mobility. Strong acids (e.g., HCl) fully dissociate, increasing conductivity, while weak acids (e.g., acetic acid) have lower conductivity due to partial dissociation.
Q: Why does acetic acid have lower electrical conductivity compared to strong acids?
A: Acetic acid is a weak acid that partially dissociates in water, producing fewer ions (H⁺ and CH₃COO⁻). Limited ion availability reduces its electrical conductivity compared to fully dissociated strong acids.
Q: How does temperature affect the electrical conductivity of acidic solutions?
A: Increasing temperature enhances ion mobility and dissociation, raising conductivity. However, excessive heat may degrade the acid or reduce solubility, potentially lowering conductivity over time.
Q: Is there a difference in electrical conductivity between concentrated and diluted acetic acid?
A: Diluted acetic acid shows higher conductivity as water facilitates partial dissociation, releasing more ions. Concentrated acetic acid has limited dissociation, resulting in lower conductivity despite higher acid content.
Q: Can electrical conductivity measurements determine acetic acid purity?
A: Yes, impurities like ions or dissolved salts alter conductivity. Pure acetic acid has low conductivity due to weak dissociation, while contaminants increase ion concentration and measurable conductivity.
Related Products
Related News


2025-05-22 16:46:14
Turbidity Test Fixtures: Advanced and Reliable Quality Assurance ToolsTurbidity, as an important indicator for measuring liquid transparency, is widely used in environmental monitoring, food and beverage production, pharmaceutical industry, and other fields.

2025-05-22 16:43:21
Total Dissolved Solids: Importance in Irrigation, Industrial Processes, and ApplicationsTotal Dissolved Solids refers to the total content of various inorganic salts and organic matter dissolved in water, and is one of the important indicators for measuring water quality.
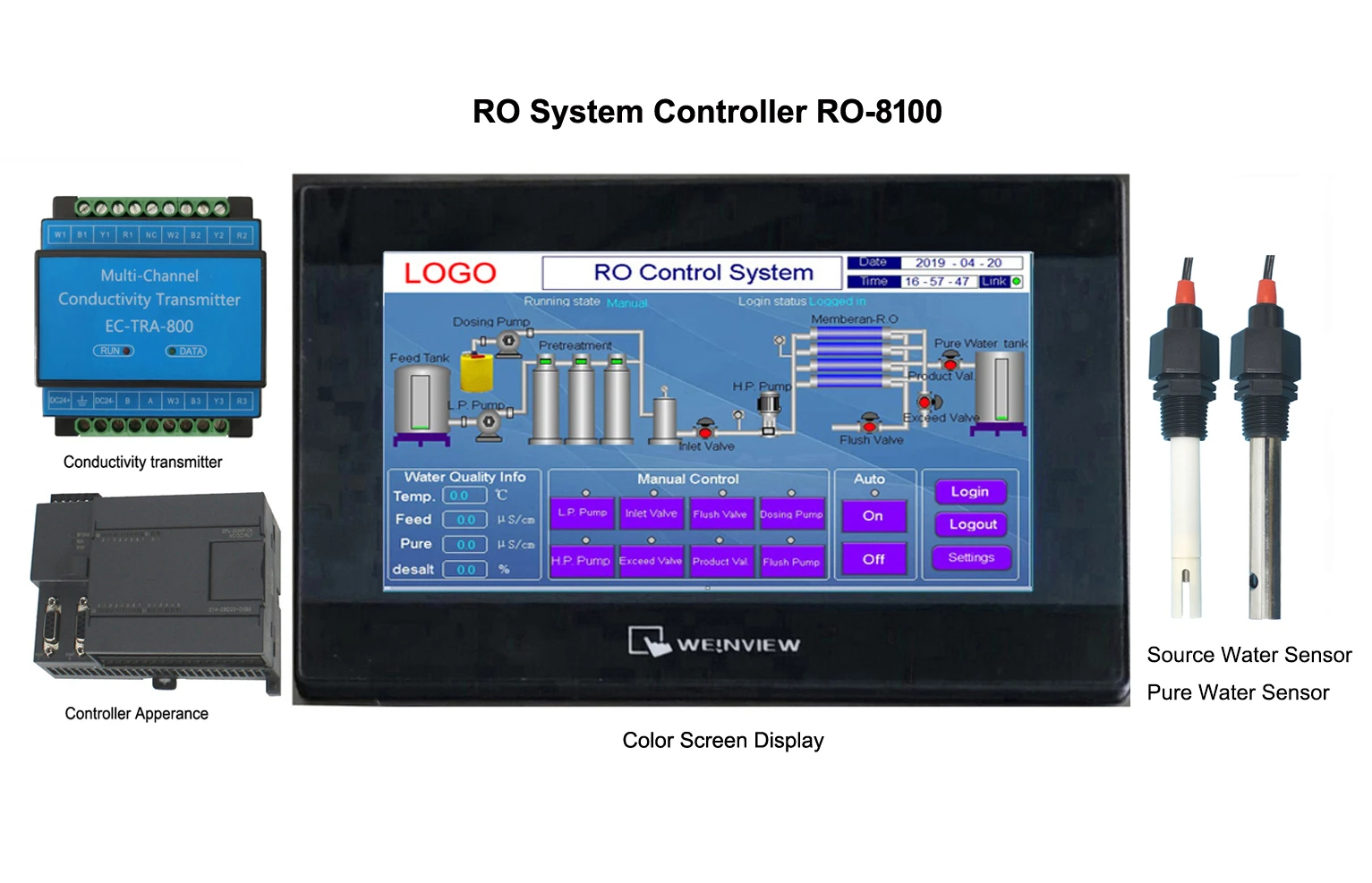
2025-05-22 16:40:50
Ro System Controller: Central nervous system in water purification systemsReverse osmosis system, as an efficient water purification technology, has been widely used in industrial, commercial, and household fields.

2025-05-22 16:37:43
Residual Chlorine Meter: A Key Guarantee for Ensuring Water Quality SafetyResidual chlorine, as an important indicator in the process of water disinfection, directly affects the safety and hygiene of drinking water and various industrial water.

2025-05-22 16:34:43
PH oORP Controller: A Key Instrument for Water Quality Monitoring and RegulationWater quality is an important indicator for measuring environmental health and industrial production.

2025-05-22 16:31:55
Dissolved Oxygen Meter: A Key Tool for Accurately Measuring Dissolved Oxygen Levels in Aquatic EnvironmentsDissolved oxygen is one of the important indicators for measuring water quality.

2025-04-21 18:03:53
Understanding Turbidity Meter Types: Which One Is Right for Your Application?Monitoring turbidity—an indicator of water clarity—is vital for applications ranging from drinking water treatment to environmental monitoring.
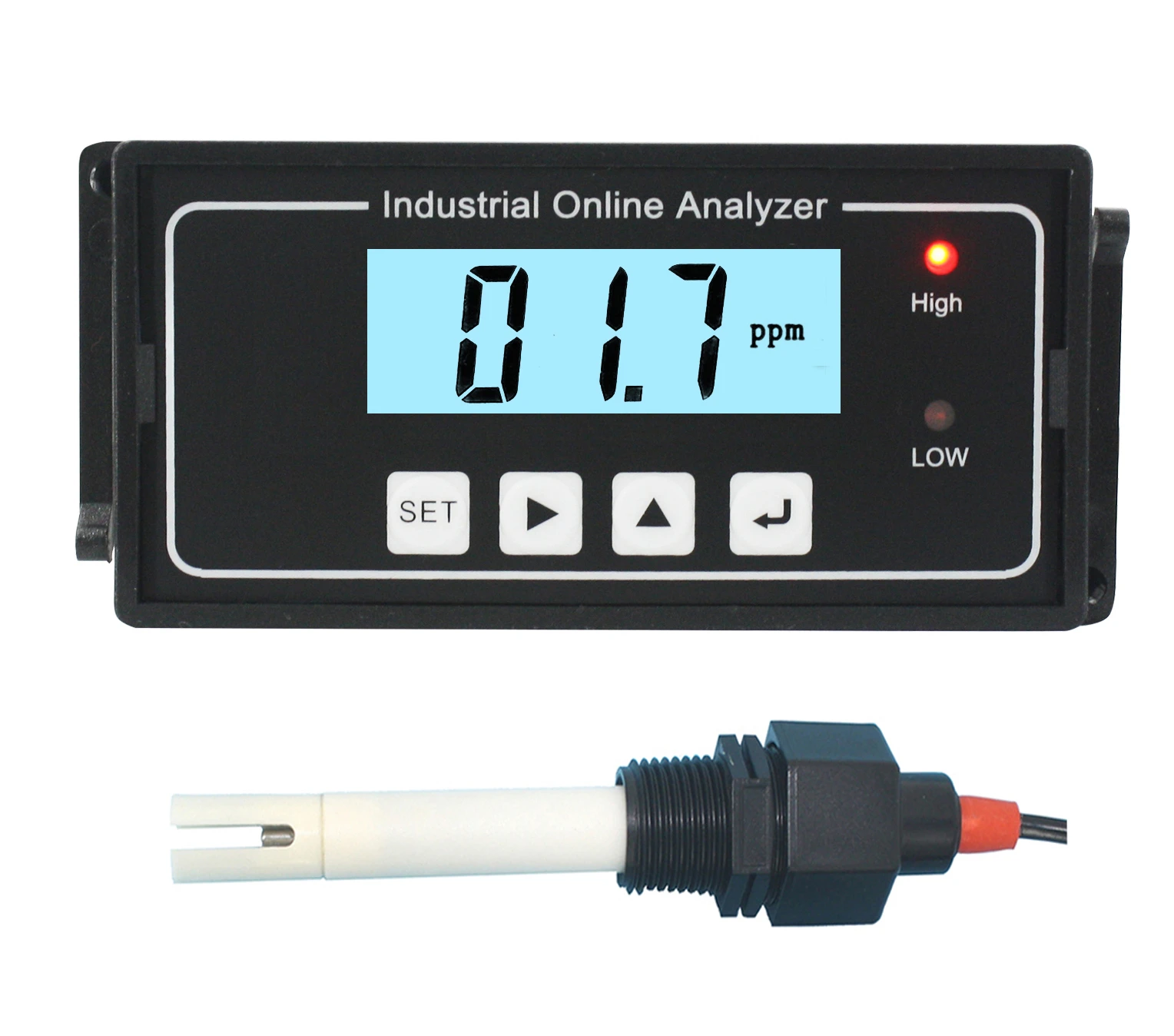
2025-04-21 18:01:21
Understanding Total Dissolved SolidsWater may look clear, but that doesn’t mean it's pure. Hidden within every glass can be a range of minerals, salts, metals, and organic substances collectively known as total dissolved solids.










