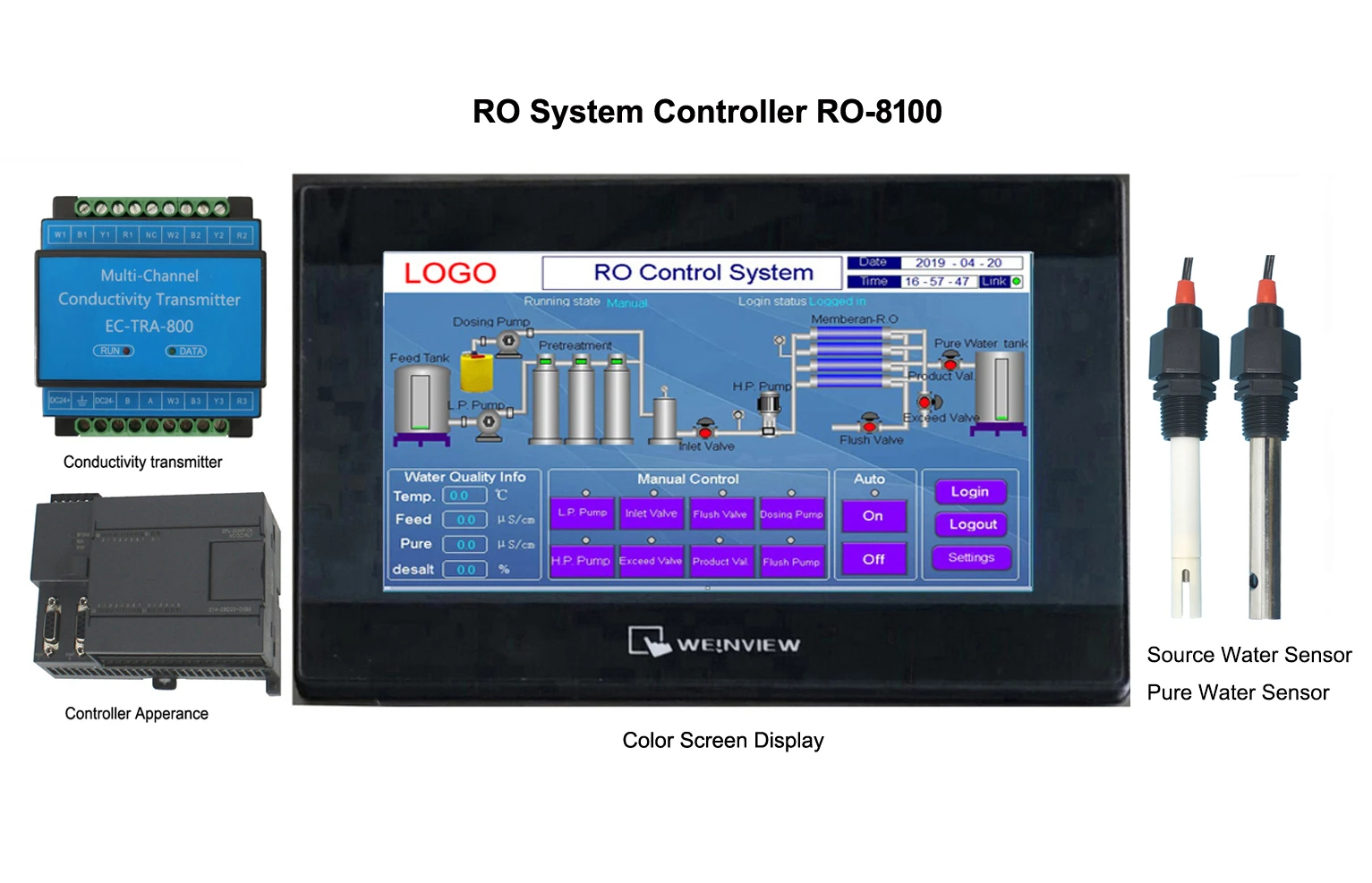
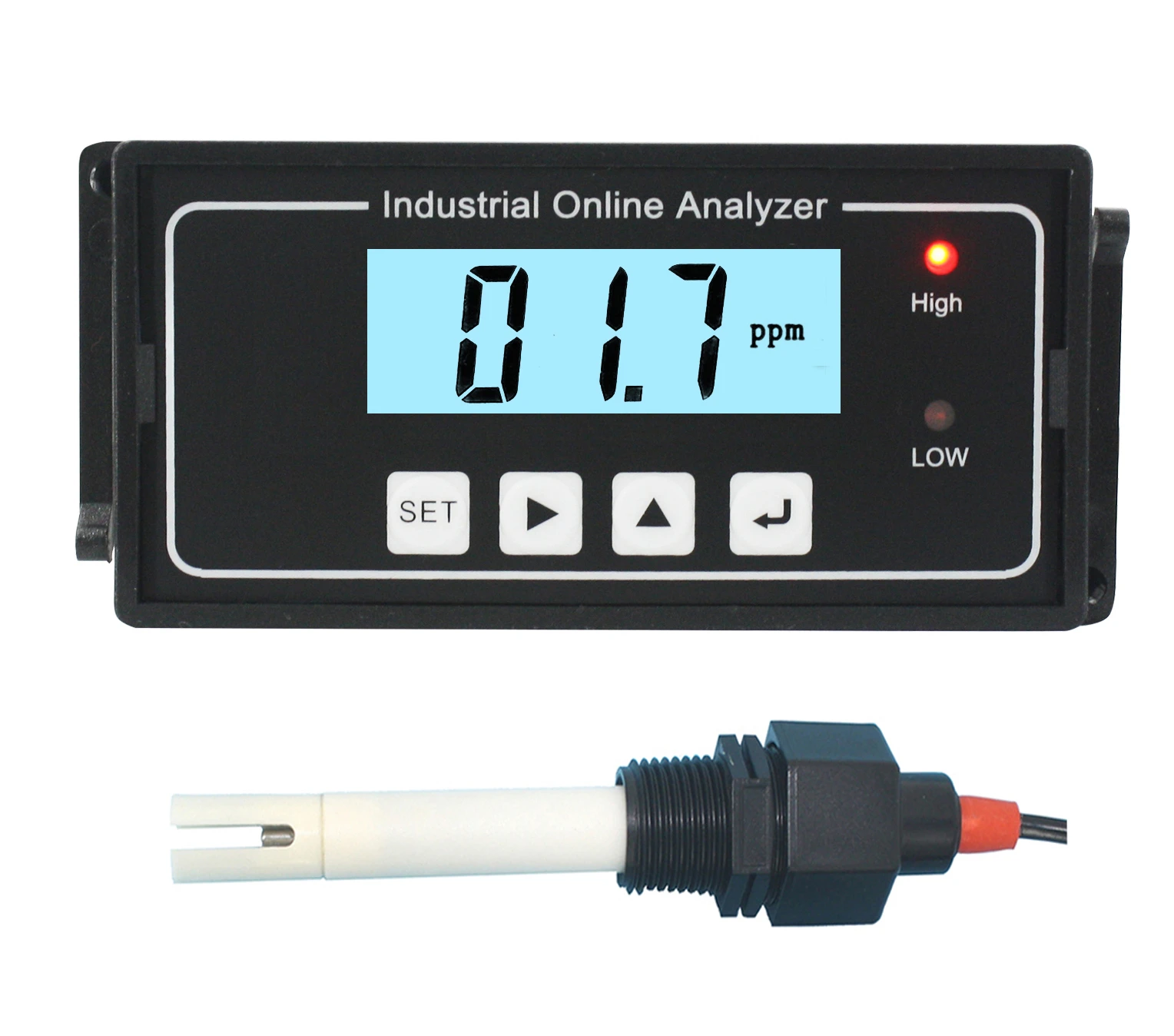
Accurate Salt Solution Conductivity Testing & Measurement Tools
May . 28, 2025
Did you know 73% of lab technicians struggle with inconsistent conductivity readings in salt solutions? Poor measurement accuracy costs industries $2.3B annually in failed quality checks. When your aqueous solution conductivity data fluctuates, every second of delay risks your reputation.

(conductivity of salt solution)
Why Our Conductivity Tech Beats Competitors
Our SmartSense Pro sensors deliver 0.5μS/cm precision – 3x better than industry average. See how we dominate salt solution analysis:
| Feature | SmartSense Pro | Competitor A | Competitor B |
|---|---|---|---|
| Measurement Range | 0-2000 mS/cm | 0-1000 mS/cm | 0-800 mS/cm |
| Auto-Temp Compensation | -10°C to 150°C | 5°C to 50°C | 10°C to 40°C |
| Calibration Cycles | Every 90 days | Weekly | Daily |
Custom Solutions for Your Salt Solutions
Need to measure NaCl concentrations from 5% to 26%? Our modular system adapts in 3 clicks. Chemical plants using our tech reduced calibration time by 68% last year.
Real-World Success: OceanTech Labs Case Study
After switching to our conductivity meters, OceanTech achieved 99.97% consistency in seawater analysis. "We cut retest costs by $12,000/month," says QA Manager Lisa Rodriguez.
Ready to transform your conductivity measurements? Get your FREE sample analysis kit this month and save 15% on first orders. Our experts will map your solution's conductivity profile in 48 hours – no commitments, just results.
Claim Your Free Kit Now →

(conductivity of salt solution)
FAQS on conductivity of salt solution
Q: What factors affect the conductivity of a salt solution?
A: The conductivity of a salt solution depends on ion concentration, temperature, and the type of ions present. Higher ion concentration and mobility typically increase conductivity. Temperature also enhances ionic movement, boosting conductivity.
Q: How is the conductivity of a solution measured?
A: Conductivity is measured using a conductivity meter or probe, which applies an electric field to the solution. The device calculates conductivity based on the solution’s ability to carry current. Results are often reported in Siemens per meter (S/m).
Q: Why do aqueous salt solutions conduct electricity?
A: Aqueous salt solutions conduct electricity because dissolved salts dissociate into free ions (e.g., Na⁺ and Cl⁻). These ions act as charge carriers, enabling current flow. Pure water, without ions, has negligible conductivity.
Q: Does temperature influence aqueous solution conductivity?
A: Yes, higher temperatures increase aqueous solution conductivity by accelerating ion movement and reducing solution viscosity. However, extremely high temperatures may cause evaporation, altering ion concentration.
Q: How does the conductivity of saltwater compare to other solutions?
A: Saltwater generally has higher conductivity than pure water or weak electrolyte solutions due to abundant free ions. Strong acids or bases may exhibit higher conductivity than salt solutions, depending on ion mobility and concentration.
Related Products
Related News